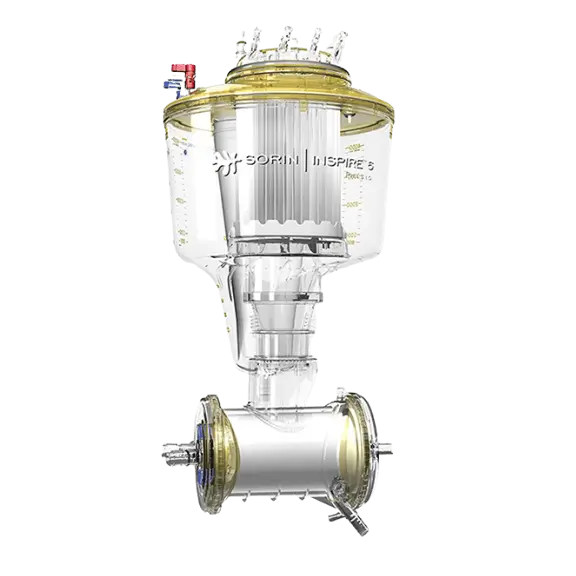
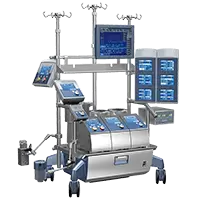
B-Capta™ Blood-Gas Monitoring
The ready-to-go in-line blood-gas monitoring system
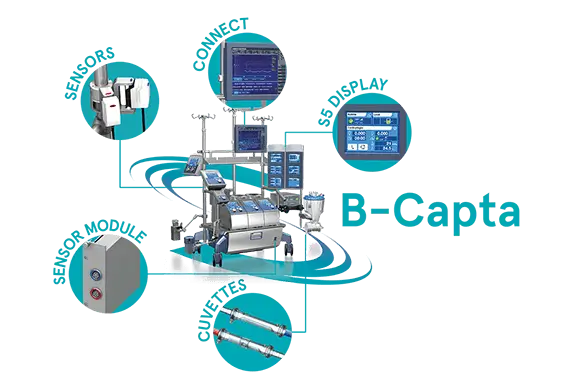
An Accurate Blood-Gas Monitoring System
Designed to easily and accurately monitor arterial and venous blood-gas parameters even in long and complex pediatric and adult cardiopulmonary bypass procedures.
Key benefits:
- Provides continuous in-line monitoring of the patient’s blood parameters and allows the operator to quickly react to changes.
- Supports the implementation of the Goal-Directed Perfusion (GDP) therapy in Connect, that enables perfusionists to maintain accurate perfusion procedure records.1,2
A Fast and Continuous Monitoring Device
Blood-gas analyzers only reflect the patient’s clinical condition at the exact moment the sample is drawn, meaning that, by the time the result is received, the blood-gas parameters no longer reflect the patient’s actual condition.3,4
By continuously monitoring the patient's blood-gas parameters, B-Capta™ can support the perfusionist during the entire cardiopulmonary bypass procedure.

Proven safe and reliable
B-Capta Sensor
B-Capta is based on an accurate and reliable optical-based technology, improved to ensure the highest level of accuracy and reliability when measuring patient blood-gas parameters, even in long and complex cardiopulmonary bypass procedures.
Accurate and continuous measurements
B-Capta allows accurate monitoring of pO2 and temperature in the arterial line and saturation, HCT/Hb and temperature in the venous line. It does not provide calculated parameters, but only measured values to ensure accurate monitoring.
Pediatric and adult procedures with the same sensor
The B-Capta arterial and venous sensors fit all disposable cuvette sizes, allowing the perfusionist to use the same sensor for both pediatric and adult procedures. They are easy to couple with the relevant disposable cuvettes as they are identified by different color-coded labels.

Ready-to-go
Sensor Module
B-Capta does not require any calibration procedure to set the device measurements, allowing the perfusionist to save time during device set up, especially in emergency cases.
Easy parameters alignment with ABL measurement
B-Capta's innovative technology embeds a compensation algorithm to easily adjust the blood-gas parameters according to those of the Hospital Laboratory Blood-Gas Analyzer (ABL) to ensure trending accuracy during the entire procedure.
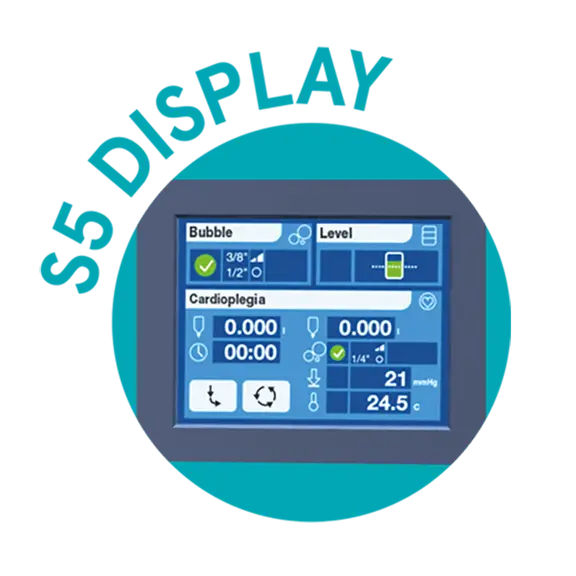
Easy and intuitive user interface
S5 Display
The venous and arterial parameters monitored by B-Capta are shown in the S5™ heart-lung machine system panel. The B-Capta user interface is intuitive and easy to use: easy parameter configuration and menu navigation, clearly displayed parameters and accessible system panel layout.
S5 user-experience
The B-Capta display is integrated in the S5 heart-lung machine allowing the perfusionist to:5
- Work without additional external monitors and holders that may obstruct the perfusionist's view during the procedure
- Have all patient and procedure parameters in the same location, reducing the levels of stress felt by the perfusionist during the procedure
Visual and audible warnings
B-Capta has an integrated warning system based on thresholds selected by the user, providing visual and audible indicators when parameters fall outside said thresholds.
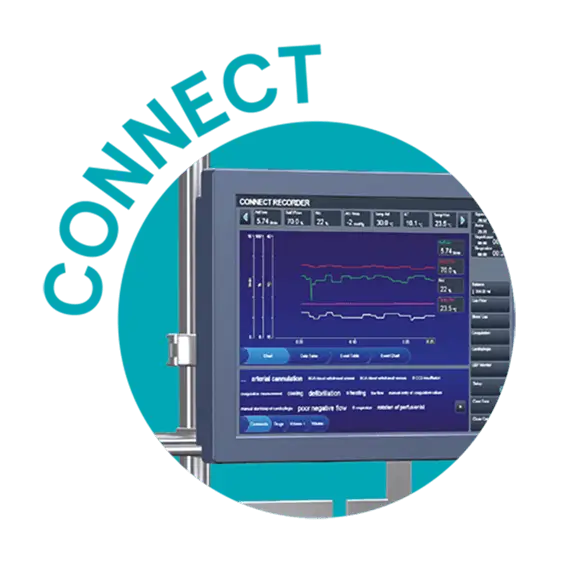
Accurate perfusion records
Connect
Throughout the entire procedure, the B-Capta blood-gas values are automatically transferred to Connect™, LivaNova’s data management system, enabling the perfusionist to maintain accurate perfusion procedure records.
Support implementation of Goal-Directed Perfusion (GDP) therapy
The B-Capta arterial and venous parameters can be used to feed the GDP formulas in Connect, as they are continuously transferred to Connect during the procedure through the S5 serial interface.1,2
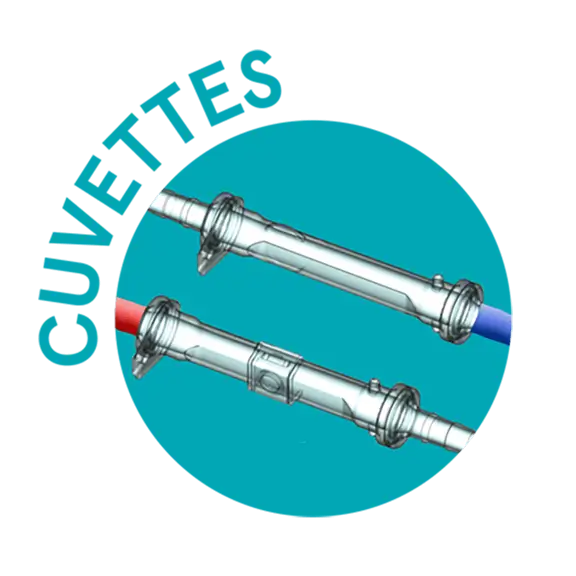
Disposable solution tailored to customers’ needs
Cuvettes
The venous and arterial disposable cuvettes are available as sterile stand-alone disposables or included in the Perfusion Tubing Systems, both pre-connected and not.
- Venous cuvettes are available in three sizes: 1/2", 1/4", 3/8"
- Arterial cuvettes are available in two sizes: 3/8", 1/4"
References
1. Ranucci M. et al., Carbon dioxide production during cardiopulmonary bypass: pathophysiology, measure and clinical relevance -Perfusion 2016.
2. RanucciM. et al., Oxygen Delivery During Cardiopulmonary Bypass and Acute Renal Failure After Coronary Operations -ATS 2005.
3. Ottens J. et al., Improving Cardiopulmonary Bypass: Does Continuous Blood Gas Monitoring Have a Role to Play? -JECT. 2010;42:191–198.
4. Trowbridge CC et al., The Effects of Continuous Blood Gas Monitoring During Cardiopulmonary Bypass: A Prospective, Randomized Study-Part II -The Journal of ExtracorporealTechnology, 2000.
5. Merkle F. at al., Evaluation of attention, perception and stress levels of clinical cardiovascular perfusionists during cardiac operations: a pilot study-Perfusion 2019
Safety Information
1. INDICATIONS FOR USE / INTENDED PURPOSE
EU: B-Capta is intended to perform, control, monitor and support extracorporeal blood circulation replacing the mechanical pumping function of the heart, monitoring and regulating physiologic parameters during procedures requiring extracorporeal circulation. The cuvettes are intended for use in adult and pediatric surgical procedures requiring cardiopulmonary to support monitoring of above mentioned parameters bypass. The devices are intended to be used for 6 hours or less. The B-Capta venous and arterial cuvettes must only be used with LivaNova equipment.
US: B-Capta is indicated for supplementary, in-line monitoring of the extracorporeal arterial oxygen partial pressure, venous oxygen saturation, venous hematocrit/hemoglobin, and arterial and venous temperature during cardiopulmonary bypass procedures up to six hours.
Canada: B-Capta is indicated for continuous, supplementary, in-line monitoring of the extracorporeal arterial oxygen partial pressure, venous oxygen saturation, venous haematocrit/haemoglobin, and arterial and venous temperature during procedures requiring extracorporeal circulation.
2. CONTRAINDICATIONS
There are no known contraindications for B-Capta.
3. WARNINGS
The device must be used in accordance with the instructions for use provided in the Instructions for Use. For a complete listing of warnings please refer to the Instructions for Use which accompany each product.
4. PRECAUTIONS
Federal law (U.S.A.) restricts this device to sale by or on the order of a physician. For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product.
The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website (www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and thorough understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Legal Manufacturer (equipment):
LivaNova Deutschland
Lindberghstrasse 25
D-80939 Munich, Germany
Legal Manufacturer (cuvettes):
Sorin group italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) Italy
Distributed in the USA by:
LivaNova USA
14401 W 65th Way
Arvada, CO 80004
For safety information of Connect and S5, please refer to relevant product pages.