Cardioplegia Products
Cardioplegia delivery sets and a cardioplegia heat exchanger for the delivery of cardioplegic solution
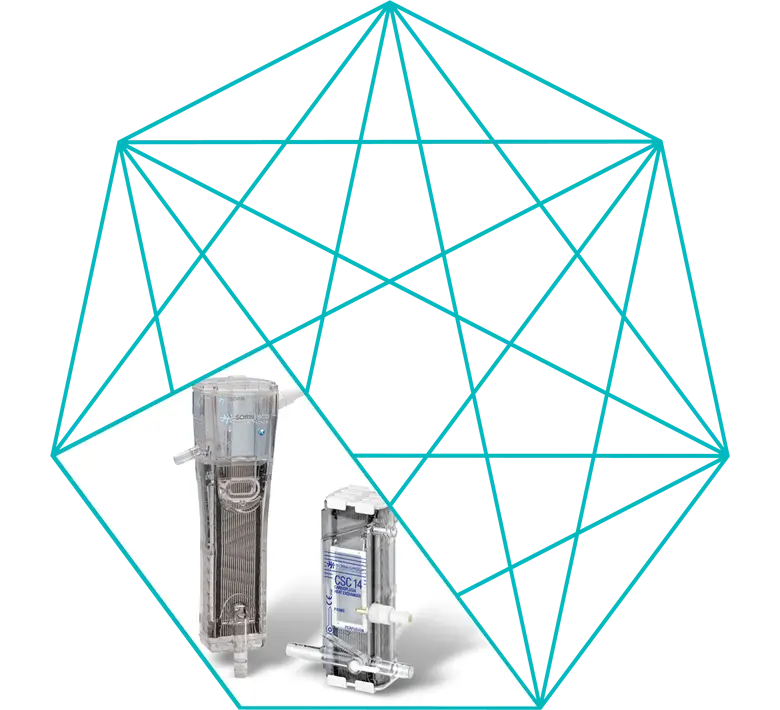
Vanguard Blood Cardioplegia Delivery Set
D921, D924, D924S, D928S, D924P are circuits designed to administer cardioplegic solution containing blood with a fixed blood/asanguineous cardioplegic solution ratio. Cardioplegic solution is used for perfusion of the coronaries during cardiopulmonary bypass, when the aorta is clamped, and the intention is to protect the myocardium during the anoxic phase.
The sets are disposable, non-toxic and non-pyrogenic and are supplier sterile in individual packs.

The sets differ in the ratios of blood to cardioplegic solution and whether or not they are equipped with shunt and luer.
- Blood compartment inlet
- Blood compartment outlet
- Filtrate outlet (UFT)
|
D924 |
4 parts blood and 1 part asanguineous cardioplegic solution |
|
D924S |
4 parts blood and 1 part asanguineous cardioplegic solution; with shunt between the blood line and the asanguineous cardioplegic solution line for administration of blood only and a luer connector on the blood line |
|
D921 |
1 part blood and 1 part asanguineous cardioplegic solution |
|
D928S
|
8 parts blood and 1 part asanguineous cardioplegic solution; with shunt between the blood line and the asanguineous cardioplegic solution line for administration of blood only and a luer connector on the blood line |
|
D924P |
4 part blood and 1 part asanguineous cardioplegic solution |
|
VANGUARD heat exchanger |
|
Safety Information
Intended Use
Vanguard Cardioplegia Sets are for the administration of cardioplegic solution containing blood, with the aid of a low flow pump.
This set allows control of the flow, the temperature and infusion (line) pressure of the cardioplegic fluid. The pump permits total control of the infusion parameters, flow and quantity of solution delivered. The perfusion temperature depends on the thermal parameters of the cold water source which supplies the VANGUARD heat exchanger.
Cardioplegia Heat Exchanger
CSC14 is a single-use stainless steel heat exchanger with integrated bubble trap.

|
|
CSC14 |
|
BLOOD FLOW RANGE |
10-2500 ml/min |
|
METAL CORE
|
|
|
BUBBLE TRAP
|
|
|
PRIMING VOLUME |
|
|
CONNECTIONS
|
|
|
SPECIAL DEVICES |
|
Safety Information
Intended Use
CSC14 is recommended for use as a heating/cooling device and bubble trap for blood cardioplegia and clear fluid perfusion inextracorporeal circulation associated with cardiopulmonary bypass. It is suggested not to use CSC14 for more than 6 hours.