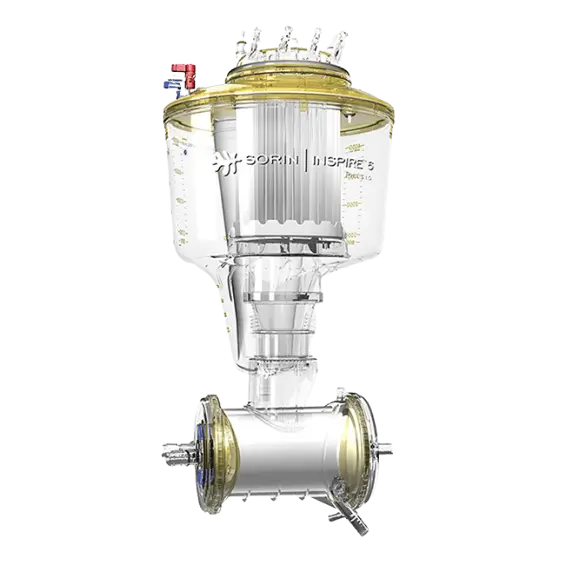
Hemoconcentrators
Hemoconcentrators with a high permeability microporous hollow fiber membrane
DHF Hemoconcentrator
The DHF hemoconcentrator is composed of a high permeability microporous hollow fiber membrane. The device is available in the following versions:
- DHF 02
- DHF 06
|
|
DHF 02 |
DHF 06 |
|
Active Surface Area |
0.25 m2 |
0.68 m2 |
|
Hollow fibre (type)
|
Polyethersulphone
|
|
|
Static priming volume (blood compartment) |
30 ml (+/-10 ml) |
60 ml (+/-10 ml) |
|
Min–Max blood flow rate (Qb) |
100-500 ml/min |
|
|
Min–Max transmembrane pressure (TMP) |
0-66 kPa (0-0.7 bar / 0-9.6 psi / 0-500 mmHg) |
|
|
Min–Max pressure drop (parameter to monitor when blood flow rate in the hemoconcentrator is not known) |
6.6-20 kPa (0.066-0.2 bar / 0.95-2.7 psi / 50-150 mmHg) |
1.3-4.6 kPa (0.013-0.046 bar / 0.91-0.67 psi / 10-35 mmHg) |
|
Blood contact materials |
Polyethersulfone; Polycarbonate; Polyurethane |
|
|
Length |
145 mm |
|
|
Diameter |
55 mm |
|
|
Weight |
98 g (0.22 lb) |
105 g (0.23 lb) |
|
Connectors:
|
|
|
Safety Information
Important Safety Information for Hemoconcentrators
1. INDICATIONS FOR USE / INTENDED PURPOSE
The device is intended for use in cardiopulmonary pypass circuit for hemoconcentration and consequent restoring of patient physiological hematrocrit. The blood to be treated should contain anticoagulant.
The choice of hemoconcentrator depends on the protocol being used and required filtration speed The transmembrane (TMP) pressure of the hemoconcentrator must always be lower than 66 Kpa ( 0.7 bar / 9.6 psi / 500 mm Hg).
All devices must be used up to 6 hours or less.
2. CONTRAINDICATIONS
The device is recommended only for use for the above-mentioned purpose. Not recommended for use in diseased or abnormal cannulation sites.
3. WARNINGS
The device must be used in accordance with the instructions for use. For a complete listing of warnings, please refer to the Instructions for Use which accompany each product.
4. PRECAUTIONS
For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product.
5. PERFORMANCE INFORMATION
The device is composed by an high permeability microporous hollow fiber membrane.
The devices are single use, non-toxic, non-pyrogenic, supplied STERILE and individually packaged. Sterilised by ethylene oxide. The level of ethylene oxide residuals in the device is within the limits established by national regulations in the country of use.
The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website ( www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Not approved in all geographies. Please consult your labeling.
Legal Manufacturer:
Sorin Group Italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) ITALY
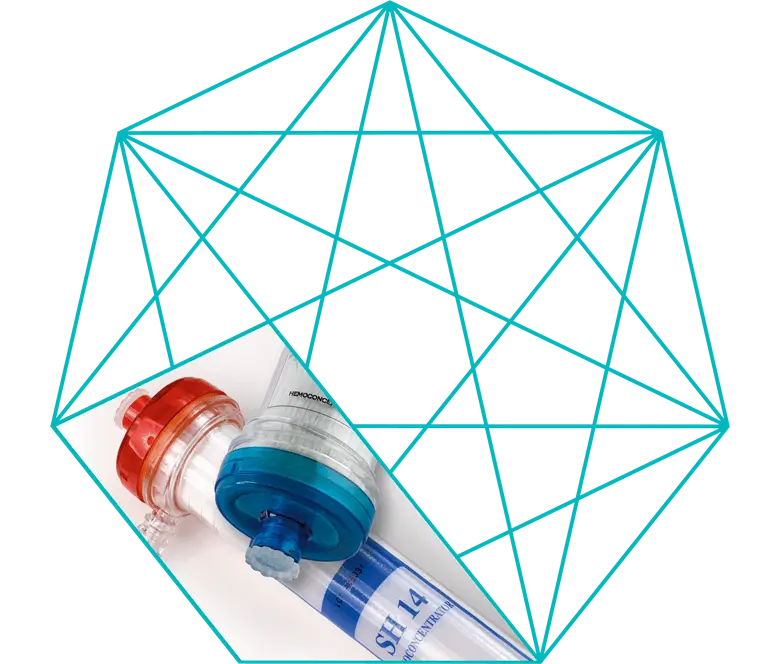
SH 14 Hemoconcentrator
The SH 14 hemoconcentrator is composed of a high permeability microporous hollow fiber membrane.
|
|
SH 14 |
|
Active Surface Area |
1.35 m2 |
|
Hollow fibre (type)
|
Polyethersulphone
|
|
Static priming volume (blood compartment) |
80 ml (+/-10%) |
|
Min-Max blood flow rate (Qb) |
100-500 ml/min |
|
Max transmembrane pressure (TMP) |
66 kPa (0.7 bar / 9.6 psi / 500 mmHg) |
|
Blood contact materials |
Polyethersulfone; Polycarbonate; Polyurethane |
|
Length |
305 mm |
|
Diameter |
39 mm |
|
Weight |
191 g (0.42 lb) |
|
Connectors:
|
|
Safety Information
Important Safety Information for Hemoconcentrators
1. INDICATIONS FOR USE / INTENDED PURPOSE
The device is intended for use in cardiopulmonary pypass circuit for hemoconcentration and consequent restoring of patient physiological hematrocrit. The blood to be treated should contain anticoagulant.
The choice of hemoconcentrator depends on the protocol being used and required filtration speed The transmembrane (TMP) pressure of the hemoconcentrator must always be lower than 66 Kpa ( 0.7 bar / 9.6 psi / 500 mm Hg).
All devices must be used up to 6 hours or less.
2. CONTRAINDICATIONS
The device is recommended only for use for the above-mentioned purpose. Not recommended for use in diseased or abnormal cannulation sites.
3. WARNINGS
The device must be used in accordance with the instructions for use. For a complete listing of warnings, please refer to the Instructions for Use which accompany each product.
4. PRECAUTIONS
For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product.
5. PERFORMANCE INFORMATION
The device is composed by an high permeability microporous hollow fiber membrane.
The devices are single use, non-toxic, non-pyrogenic, supplied STERILE and individually packaged. Sterilised by ethylene oxide. The level of ethylene oxide residuals in the device is within the limits established by national regulations in the country of use.
The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website ( www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Not approved in all geographies. Please consult your labeling.
Legal Manufacturer:
Sorin Group Italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) ITALY

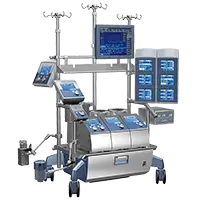
Essenz™ Perfusion System
Enter the New Era of Perfusion
Built on LivaNova’s 50-year legacy of safety and reliability, Essenz supports the Perfusionist in doing what is best for each patient, and allows the entire heart team to continuously improve their clinical practice.
Please Note: Essenz information available only in selected geographies.