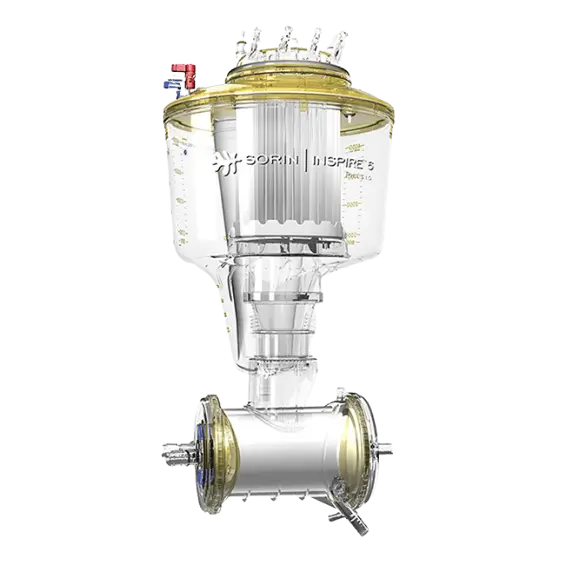
Xtra™ Autotransfusion System
The extraordinary flexible, intuitive and powerful ATS System


Xtra™ autotransfusion system is the complete solution for blood management.
It brings together three decades of experience and improvements in cell salvage procedures, treating more than 0.5 million patients per year.
Ready to meet any surgical scenario: from scheduled treatments to emergency situations and everything in between
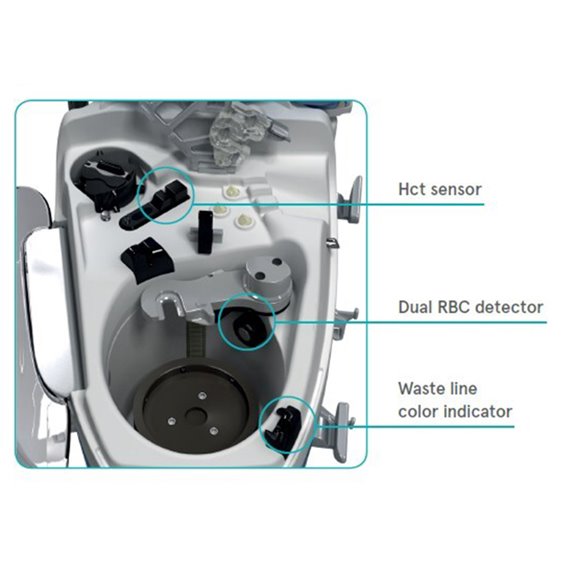
Performance where you need it1
- Consistently high RBC hematocrit and wash quality with standard protocol
- Excellent heparin and protein removal
- High RBC recovery rate
- Quiet and powerful vacuum pump
- Specific protocol for fat removal
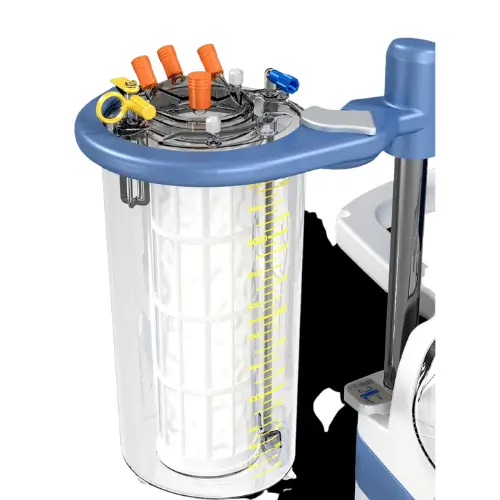
Flexibility where you want it 2
- Flexible choice of intra, post-op and sequestration protocol
- Wide range of bowl sizes and disposables to choose from
- Low minimum required Red Blood Cell mass to process
- Fast Processing to manage high volume blood loss
- Complete control over your data, printed or stored
- Emergency protocol for fast blood process
.webp?language=en-US)
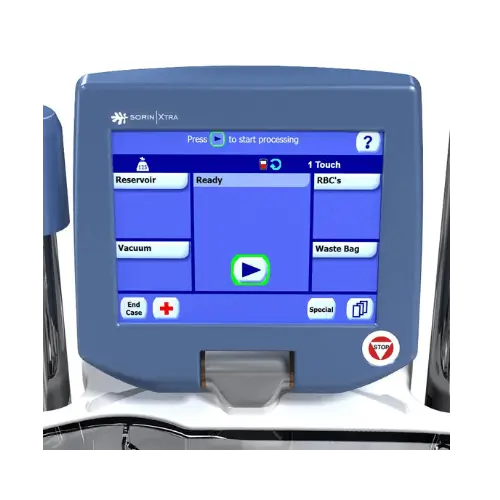
Intuitive when you use it
- Fast and intuitive setup
- Fully automated operation from the start to the end of the caseIntuitive
- User Interface with color touch screen, mimicking the S5 and C5 Heart-Lung Machines controls
- Refined ergonomics and reduced footprint for manageable procedures
Clinical Applications
Despite being a widely used procedure, there are known risks associated with blood transfusion. Adverse events are still found in clinical practice related either directly or indirectly to homologous blood transfusion.3,4
Autotransfusion plays a key role as part of an effective blood management strategy.5 It can contribute to improved clinical outcomes in a cost-effective manner, either as stand-alone or as part of a Patient Blood Management program.6-8
In Cardiac Surgery
Patients undergoing cardiac surgeries are commonly at an advanced stage and comorbidities such as myocardial infarction, making effective blood products management an essential component of their care.9-11
Autotransfusion lowers the transfusion rates during and after Cardiac Surgery.7,8
Outside of Cardiac Surgery
The rate of allogeneic red blood cell transfusions in non-cardiac surgery settings has been found to be between 21% and 70%, with the majority of authors reporting figures in the middle of the range.12 Allogeneic blood transfusion raises multiple challenges:13
- Risk of pathogen transmission
- Health/economic costs
- Scarcity of resources
Autotransfusion supports clinicians and patients in reducing blood transfusions.4 What’s more, it is a cost-effective option in different surgical fields.8
- In orthopedics, autotransfusion is safe, effective and reduces the risk of transmission of infections.1
- In vascular surgery, it reduces the risk of allogeneic blood transfusion, the length of intensive care unit and hospital length stay.15-17
- In obstetric surgery, autotransfusion is considered to be more effective and useful than the other blood conservation techniques and endorsed by CEMACH, OAA/AAGBI guidelines, the National Blood Service and NICE.16
- In transplant surgery, autotransfusion can reduce the need for heterologous blood transfusion and the risk of transmissible diseases.19,20
.webp?language=en-US)
References
1. Overdevest et al. "Clinical evaluation of the Sorin Xtra® autotransfusion system." Perfusion 2012, 27(4) 278–283
2. Bauman et al. (2015) "Evaluation of the minimum volume of salvaged blood required for the successful use of two different autotransfusion device" Pediatric Anesthesia 25 (2015) 258–264
3. Kilic et al. "Blood transfusions in cardiac surgery: indications, risks, and conservation strategies." Ann Thorac Surg. 2014 Feb;97(2):726-34.
4. Kozek-Langenecker et al. “Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology." Eur J Anaesthesiol 2017; 34:332–395
5. Meybohm et al. "Washed cell salvage in surgical patients. A review and meta-analysis of prospective randomized trials under PRISMA." Medicine (Baltimore). 2016 Aug;95(31):e4490
6. Roberts et al. "The global need and availability of blood products: a modelling study." Lancet Haematol. 2019 Dec;6(12):e606-e615.
7. Vonk et al. "Intraoperative cell salvage is associated with reduced postoperative blood loss and transfusionrequirements in cardiac surgery: a cohort study." Transfusion. 2013 Nov;53(11):2782-9.
8. Côté et al. "Efficacy of intraoperative cell salvage in decreasing perioperative blood transfusion rates in first-time cardiac surgery patients: a retrospective study." Can J Surg. 2016 Sep;59(5):330-6.
9. Robich et al. "Trends in blood utilization in United States cardiac surgical patients." Transfusion. 2015 Apr;55(4):805-14.
10. Geissler et al. "Utilisation of blood components in cardiac surgery: a single-centre retrospectiveanalysis with regard to diagnosis-related procedures." Transfus Med Hemother . 2015 Mar;42(2):75-82.
11. Ad et al. "Effect of patient age on blood product transfusion after cardiac surgery." The Journal of Thoracic and Cardiovascular Surgery, 19 Mar 2015, 150(1):209-214
12. Carling et al."Transfusions and blood loss in total hip and knee arthroplasty: a prospective observational study." J Orthop Surg Res. 2015 Mar 28;10:48.
13. Carless et al. "Cell salvage for minimising perioperative allogeneic blood transfusion." Cochrane Database Syst Rev. 2010 Mar 17;(3):CD001888. doi: 10.1002/14651858.CD001888.pub3. Review. Update in Cochrane Database Syst Rev. 2010;(4):CD001888.
14. Shenolikar A, et al. "Cell salvage auto transfusion in total knee replacement surgery." Transfus Med. 1997;7:277–80.
Safety Information
Summary of Safety & Performance Information for ATS (equipment & disposables)
1. INDICATIONS FOR USE / INTENDED PURPOSE
The XTRA is intended to be used within the XTRA system for preoperative sequestration, intraoperative cell
salvage, and/or postoperative cell salvage, aimed at autotransfusion.
The use of XTRA equipment for intraoperative cell salvage is intended to collect and process aspirated blood
from the surgical field and concentrate it in red blood cells.
The use of XTRA equipment for postoperative cell salvage is intended to collect and process blood from
wound drains and concentrate it in red blood cells.
The use of XTRA equipment for pre-operative sequestration is intended to process whole blood and separate
it into platelet-poor plasma (PPP) and/or platelet-rich plasma (PRP) and red blood cells.
The XVAC is intended to be used within the XTRA system for vacuum application during intraoperative and/or
post-operative cell salvage, aimed at autotransfusion.
The use of the device for intraoperative cell salvage is intended to aspirate blood from the surgical field for
subsequent blood processing.
The use of the device for post-operative cell salvage is intended to aspirate blood from surgical wound drains
for subsequent blood processing.
The XTRA BOWL SET is intended to be used within the XTRA system for preoperative sequestration,
intraoperative cell salvage, and/or postoperative cell salvage, aimed at autotransfusion.
The use of the device for intraoperative cell salvage is intended to process aspirated blood from the surgical
field and concentrate it in red blood cells.
The use of the device for postoperative cell salvage is intended to process blood from wound drains, and
concentrate it in red blood cells.
The use of the device for pre-operative sequestration is intended to process whole blood and separate it
into platelet and red blood cells.
The SEQUESTRATION SET X is intended to be used within the XTRA system for preoperative sequestration,
aimed at autotrasfusion. The use of the device for preoperative sequestration is intended to separate whole
blood into 0platelet poor plasma (PPP) and/or platelet rich plasma (PRP) and Red blood cells.
The XTRA XRES BLOOD COLLECTION RESERVOIRS and XTRA COLLECTION SETS are intended to
be used within the XTRA system for intraoperative and/or post-operative cell salvage, aimed at
autotransfusion.
The use of XRES Blood Collection Reservoirs and Collection Sets for Intraoperative cell salvage is
intended to collect and filter aspirated blood from the surgical field.
The use of XRES Blood Collection Reservoirs and Collection Sets for postoperative cell salvage is
intended to collect and filter blood from wound drain.
The XTRA ACCESSORIES are intended to be used within the Xtra system:
- The vacuum extension line is intended to connect the XRES collection reservoir to Xvac equipment or any other vacuum source.
- The blood reinfusion bag is intended to store the processed blood or blood components for subsequent
- reinfusion to the patient.
- The waste bag is intended to collect the fluids and cellular debris removed from the processed blood.
- The Xres B Reservoir “Y” Adaptor is intended for connection between the Xres Reservoir (bottom version) and the extra-corporeal circulation (ECC) circuit.
- The Cardio Kit is intended for connection between the Xres Reservoir (top version) and the extra-corporeal circulation (ECC) circuit.
- The Tandem Reservoir “Y” connects two Blood Collection Reservoirs to a wash set of Xtra system.
- Multidiameter adapter and 4-way adapter are intended to connect lines of different sizes to the Xtra system
- Aspiration and anticoagulation lines are intended to transfer aspirated blood from the operating field to a Blood Collection Reservoir and mix it with the anticoagulant solution.
All the XTRA accessories are used for intraoperative and post-operative cell salvage, aimed at
autotransfusion. The Blood Reinfusion Bag and the Waste Bag can be also used for preoperative
sequestration, aimed at autotransfusion.
The GOCCIA filters are intended to remove microaggregates during the administration of autologous blood,
and blood products and non-blood fluids to the patient or CPB circuit.
IINDICATIONS FOR USE for UNITED STATES Only
The XTRA Autotransfusion System (including the XTRA BOWL SYSTEM, SEQUESTRATION SET X,
XTRA XRES BLOOD COLLECTION RESERVOIRS, XTRA COLLECTION SETS and XTRA
ACCESSORIES) is indicated for intraoperative recovery of blood, washing of blood collected in the
postoperative period, and preoperative sequestration (with indirect patient connection). Typical clinical
applications of autotransfusion include the following surgical specialties:
- Cardiovascular
-Orthopedics
-Thoracic
-Transplant Surgery
-Emergency (Trauma)
-Neurosurgery
-Obstetrics and gynecology
-Urology
2. CONTRAINDICATIONS
For intra and Post-operative cell salvage (XTRA; XVAC, XTRA BOWL SYSTEM, XTRA XRES BLOOD
COLLECTION RESERVOIRS, XTRA COLLECTION SETS and XTRA ACCESSORIES.
There are no known absolute contraindications for the use of XTRA system. However, potential contamination of the aspirated blood with bowel contents, infection or tumoral cells should be regarded as a relative contraindication for the reinfusion of salvaged blood, depending on the likelihood/degree of contamination. In these situations, an assessment of the risks and benefits of reinfusion of the salvaged blood should occur.
For Pre-operative Sequestration (XTRA, BOWL SET SEQUESTRATION SET X and XTRA ACCESSORIES)
The absolute contraindications are platelet disfunction syndrome and critical thrombocytopenia. The relative
contraindications are consistent use of Non-Steroid Anti-inflammatory Drug within 48 hours of procedures,
platelet count lower than 105/ul and patient under antiplatelet medication.
For GOCCIA filters.
No contraindications are known if the device is used for the purpose described and in accordance with the
stated operating conditions. Do not use the device for any purpose other than indicated.
3. WARNINGS
The device must be used in accordance with the instructions for use provided. For a complete listing of
warnings please refer to the Instructions for Use which accompany each product.
4. PRECAUTIONS
Federal law (U.S.A.) restricts this device to sale by or on the order of a physician. For a complete listing of
precautions/cautions please refer to the Instructions for Use which accompany each product.
5. ADVERSE EVENTS
The following list summarizes harms potentially arising during the use of the medical device, including those related to side effects: Allergic reactions, Bleeding, Cancer, Cyto-toxic reactions, Embolism / Thrombosis, Embolism, Environment contamination, Genetic mutation, Hemolysis, Hypersensitivity reactions, Infection (User or Other persons), Inflammatory reaction, Irritation, Sepsis / Infection, Skin tears (User or Other persons), Systemic Inflammatory Response Syndrome (SIRS).
6. PERFORMANCE INFORMATION
The devices are designed to come into contact with patient blood and are single use, non-toxic, non-pyrogenic, supplied sterile in individual packaging. Sterilised by ethylene oxide. The level of ethylene oxide residuals in the device is within the limits established by national regulations in the country of use. The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website (www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Legal Manufacturer Equipment:
LivaNova Deutschland GmbH
Lindberghstrasse 25
D-80939 Munich Germany 41037
Legal Manufacturer Disposables:
Sorin Group Italia S.r.l
Via Statale 12 Nord, 86
Mirandola (MO) Italy
Distributed in the USA by:
LivaNova USA
14401 W 65th Way
Arvada, CO 80004
Accessories Catalog

Essenz™ Perfusion System
Enter the New Era of Perfusion
Built on LivaNova’s 50-year legacy of safety and reliability, Essenz supports the Perfusionist in doing what is best for each patient, and allows the entire heart team to continuously improve their clinical practice.
Please Note: Essenz information available only in selected geographies.