Complete the form to stay in touch or get a call back from a LivaNova representative.

Discover the #NewEraofPerfusion
Join Our Mailing List Stay up-to-date with the latest Essenz Perfusion System information.
Join Our Mailing List

Essenz Perfusion System combines the state-of-the-art Essenz HLM with an intuitive Patient Monitor and accurate sensing technology, enabling data-driven decision making.
Safe and Reliable
Patient-Tailored
Intuitive
Flexible and Efficient
The Essenz™ Perfusion System is built on a 50-year legacy of trusted partnership with perfusionists. It is designed to meet evolving standards of safety and reliability, to deliver life-saving care to patients.

Innovation starts from an unmatched foundation:
State-of-the-art safety precautions:

State-of-the-art on-site support and escalation process:
The combination of accurate sensing technology, the mast mounted pumps and embedded clinical logic allows perfusionists to deliver patient-tailored perfusion therapies
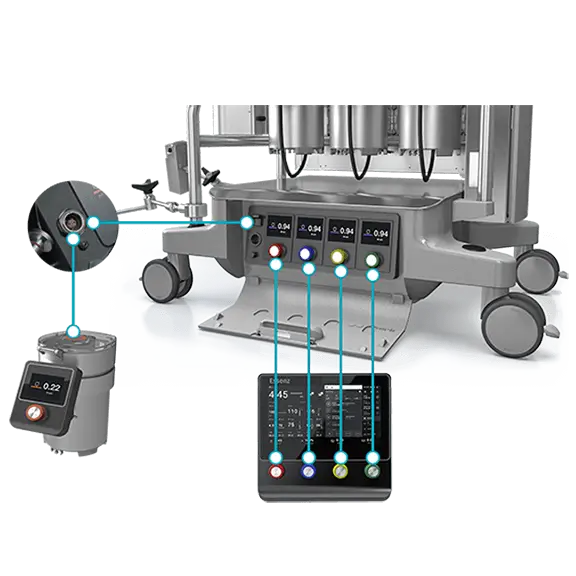
Accurate sensing technology and official formulas to support the implementation of Goal-Directed Perfusion (GDP) therapy:1
1. Goal-Directed Perfusion to reduce Acute Kidney Injury: A Randomized Trial Ranucci M. et al. J Thorac Cardiovasc Surg. 2018 Nov;156(5):1918-1927.e2. doi.org/10.1016/j.jtcvs.2018.04.045
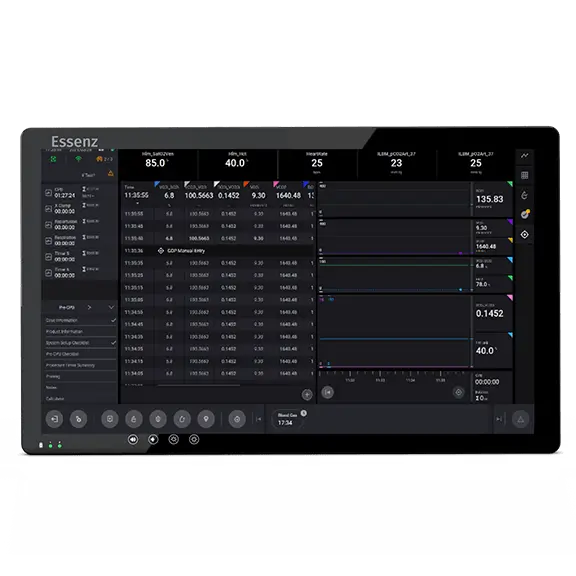
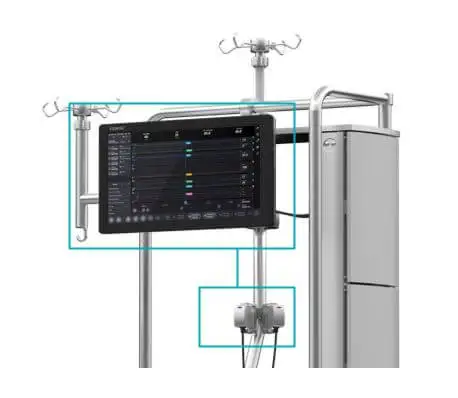
Patient Monitor with embedded clinical logic to support data-driven clinical decision making:
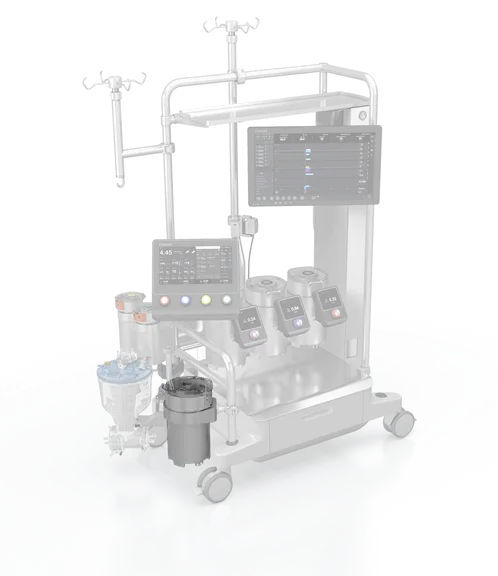
Modular system:
2. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. - Wan S, LeClerc JL, Vincent JL.Chest. 1997 Sep;112(3):676-92. doi: 10.1378/chest.112.3.676.PMID: 9315800
The Essenz Perfusion System supports clinical requirements through a combination of state-of-the-art technology and a user-friendly experience, designed and validated with perfusionists.
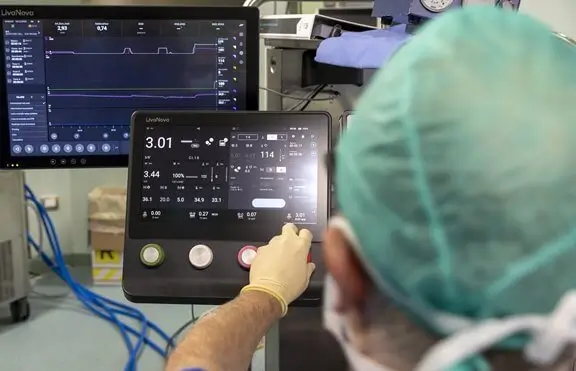
[Image: San Donato Hospital, Italy]
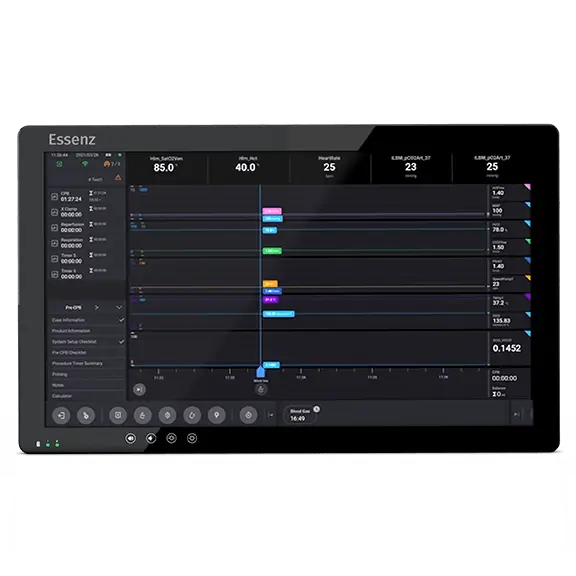

The fully integrated Essenz Perfusion System enables great efficiency in managing the procedure and connects to the wider hospital infrastructure.

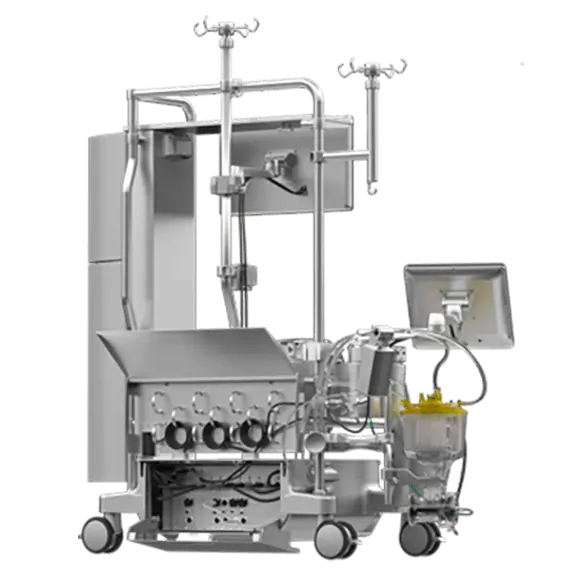
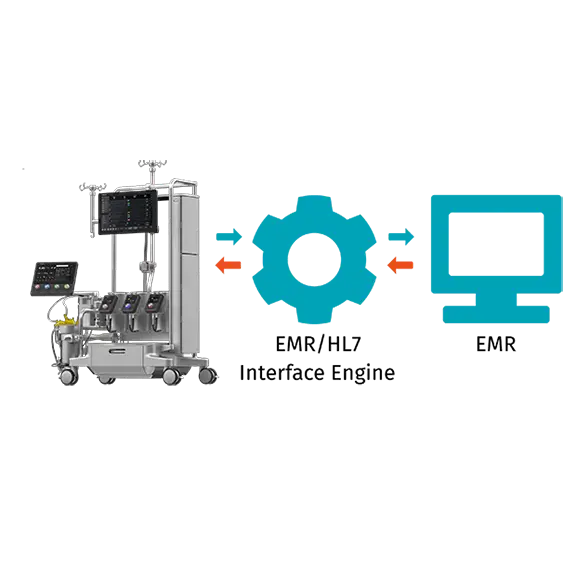
Meet our integrated perfusion system that provides value both inside and outside the OR. The system includes:
built on LivaNova’s 50-year legacy of safety and reliability
Complete the form to stay in touch or get a call back from a LivaNova representative.
The Essenz Heart-Lung Machine is intended to perform, control, monitor and support extracorporeal blood circulation replacing the mechanical pumping function of the heart, monitoring and regulating physiologic parameters during procedures requiring extracorporeal circulation.
US: Essenz HLM is intended to be used during cardiopulmonary bypass for procedures lasting six (6) hours or less.
Essenz ILBM is indicated for supplementary, in-line monitoring of the extracorporeal arterial oxygen partial pressure, venous oxygen saturation, venous hematocrit/hemoglobin, and arterial and venous temperature during cardiopulmonary bypass procedures up to six hours.
Essenz Patient Monitor
The Essenz Patient Monitor software is a modularly structured software program package that is exclusively used with LivaNova heart-lung machines. The system allows detailed recording of perfusion data during cardiopulmonary bypass procedures as well as the processing and evaluation of this data. The data may be recorded automatically or entered manually. The LivaNova Perfusion System Monitor is a panel PC intended to be exclusively used with LivaNova heart lung machines as a base and user interface for the Essenz Patient Monitor software. Essenz Patient Monitor is an FDA Cleared medical device.
CONTRAINDICATIONS: No contraindications are known if the devices are used for the purpose described and in accordance with the stated operating conditions. Do not use the devices for any purpose other than indicated.
WARNINGS: The devices must be used in accordance with the instructions for use provided. For a complete listing of warnings please refer to the Instructions for Use. Medical intervention and therapeutic procedure must not be based solely on the Essenz Patient Monitor perfusion records (reports).
PRECAUTIONS: Federal law (U.S.A.) restricts these devices to sale by or on the order of a physician. For a complete listing of precautions/cautions please refer to the Instructions for Use. The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer.
Essenz In-Line Blood Monitor and B-Capta Blood Gas Monitor are clinically equivalent in terms of safety and performance as they have the same technology and intended use. Not approved in all geographies, consult your labeling. Please visit the LivaNova website to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse.
Legal Manufacturers:
LivaNova Deutschland
Lindberghstrasse 25
D-80939
Munich, Germany
Sorin group italia S.r.l.
Via Statale 12 Nord, 86
Mirandola (MO) Italy
Distributed in the US by:
LivaNova USA
14401 W 65th Way
Arvada, CO 80004, USA
Product Disclaimer: For professional use. Not approved in all geographies. Consult the labeling for your country.
You are about to leave the LivaNova site and will now visit our Funding Requests portal. Please login or create an account to submit your application.