Electronic Gas Blender
Providing a continuous comparison of actual and set values
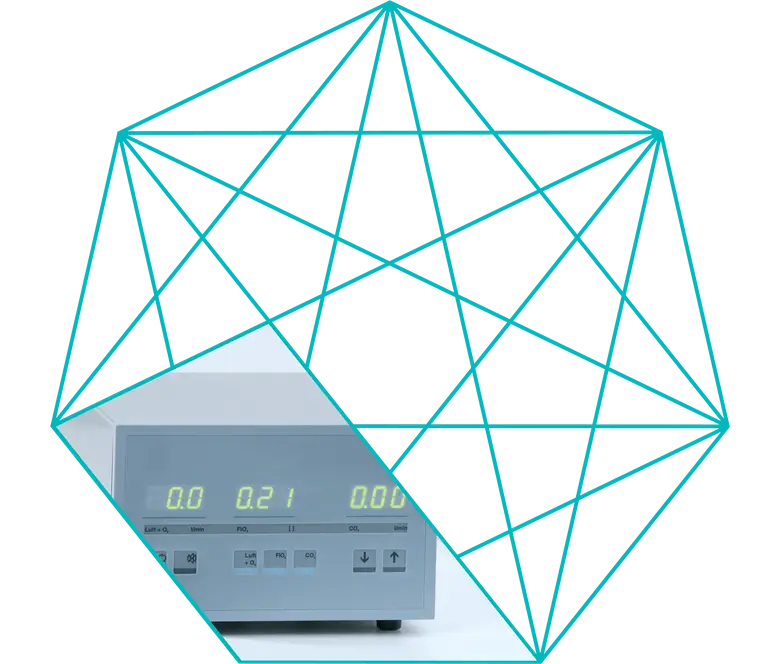
Continuous comparison of actual and set values
In the Electronic Gas Blender, actual and set values are continuously compared.
The actual value is measured by two independent sensors and an alarm is triggered if a deviation between the two values is detected.
The remote control of the Electronic Gas Blender can be used tochange the set values for Air + O2, FiO2 and CO2, from gas flow to blood flow. The operator is made aware of the actual values exceeding or dropping below the set values by acoustic and visual signals.
The Electronic Gas Blender is available in three different versions:
- Electronic Gas Blender (10 l/min) for adult perfusion
- Electronic Gas Blender (5 l/min) for pediatric perfusion
- Electronic Gas Blender (2 l/min) for infant/neonate perfusion

Safety Information
1. INDICATIONS FOR USE / INTENDED PURPOSE
EU: The gas blender is designed and intended to be used, in accordance with the above listed standards and regulations, to precisely set, monitor and control gas flows (air/O2/CO2). The gas mixture serves to oxygenate the blood in an oxygenator.
USA: The Stöckert Gas Blenders are intended to enable qualified personnel to set, monitor and control gas flows of medical grade gases (air/O2/CO2) during cardiopulmonary bypass. The Stöckert Gas Blenders are used as a component part of or optional accessory to the Stöckert S5 System (or any compatible system using the S5 firmware versions of 3.0 or greater) for periods of six hours or less.
CAN: The Stöckert Gas Blender is used with a Stöckert/Sorin S5/C5 heart-lung machine to set, monitor and control gas flow during extracorporeal perfusion.
2. CONTRAINDICATIONS
There are no known contraindications for the gas blender. The attending physician is solely responsible for the use of the system.
3. WARNINGS
The device must be used in accordance with the instructions for use provided in the Instructions for Use. For a complete listing of warnings, please refer to the Instructions for Use which accompany each product
4. PRECAUTIONS
Federal law (U.S.A.) restricts this device to sale by or on the order of a physician. For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product
The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website (www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Legal Manufacturer:
LivaNova Deutschland
Lindberghstrasse 25
D-80939 Munich, Germany
Distributed in the USA by:
LivaNova USA
14401 W 65th Way
Arvada, Colorado 80004

Essenz™ Perfusion System
Enter the New Era of Perfusion
Built on LivaNova’s 50-year legacy of safety and reliability, Essenz supports the Perfusionist in doing what is best for each patient, and allows the entire heart team to continuously improve their clinical practice.
Please Note: Essenz information available only in selected geographies.