LifeSPARC Information Hub
We aim to support our customers as the Advanced Circulatory Support Business Unit winds down. Educational resources and key information can be found below or contact us for additional assistance.
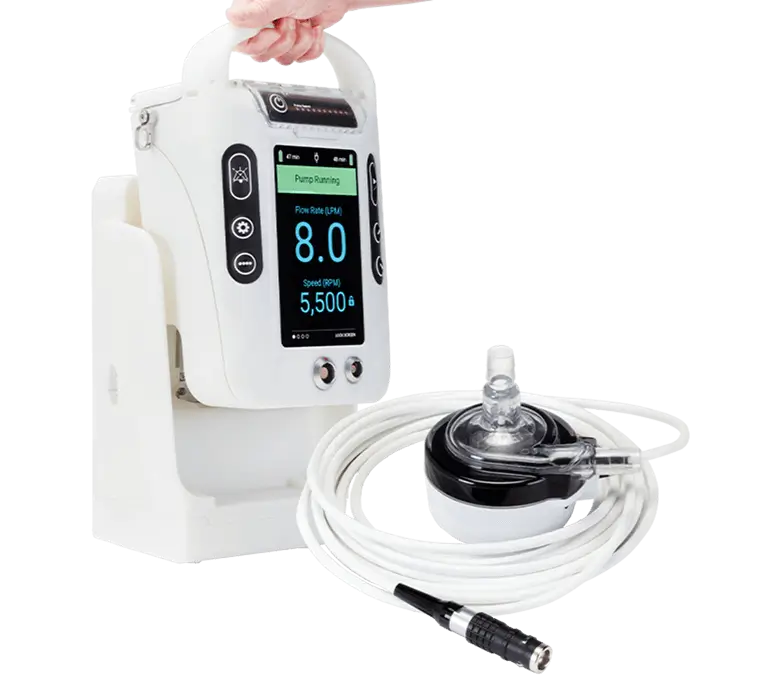
We aim to support our customers as the Advanced Circulatory Support Business Unit winds down. Educational resources and key information can be found below or contact us for additional assistance.
