Medication alone is not enough when managing drug-resistant epilepsy

Patients with poorly controlled epilepsy are at more than a 10× greater risk of premature death versus those who achieve seizure freedom1

ASM intolerance is one of the strongest and most consistent negative predictors of QoL for patients with DRE and is associated with behavioral and adverse events2,4

Rates of intolerable adverse effects and seizure freedom with ASMs have been stagnant despite 12+ medications being approved in the last 30 years5,6
VNS Therapy™ has been used to deliver life-changing treatment to more than 175,000 people with drug-resistant epilepsy, including 50,000+ children3
.png?language=en-US)
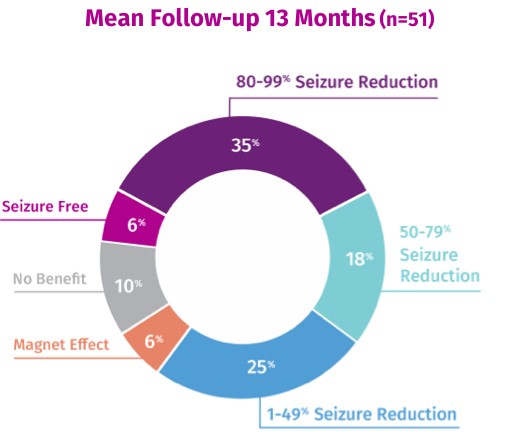
VNS Therapy™ Can De-Intensify Seizures and Provide Improvements in Seizure Control6*
59% reported ≥ 50% reduction in seizures
41% reported ≥ 80% reduction in seizures
* Data is from devices with AutoStim Mode enabled, which is only available in models 106, 1000, and 1000-D.
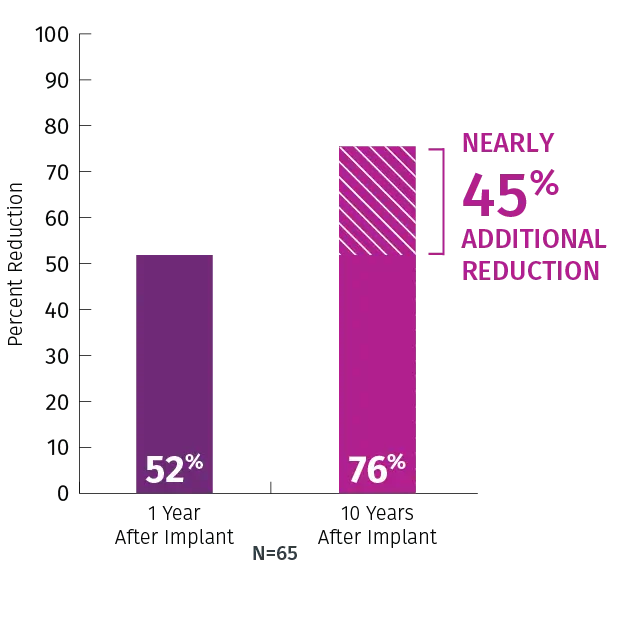
VNS TherapyTM response improves over time with a 76% mean reduction in seizures at 10 years
Mean reduction in seizure frequency8

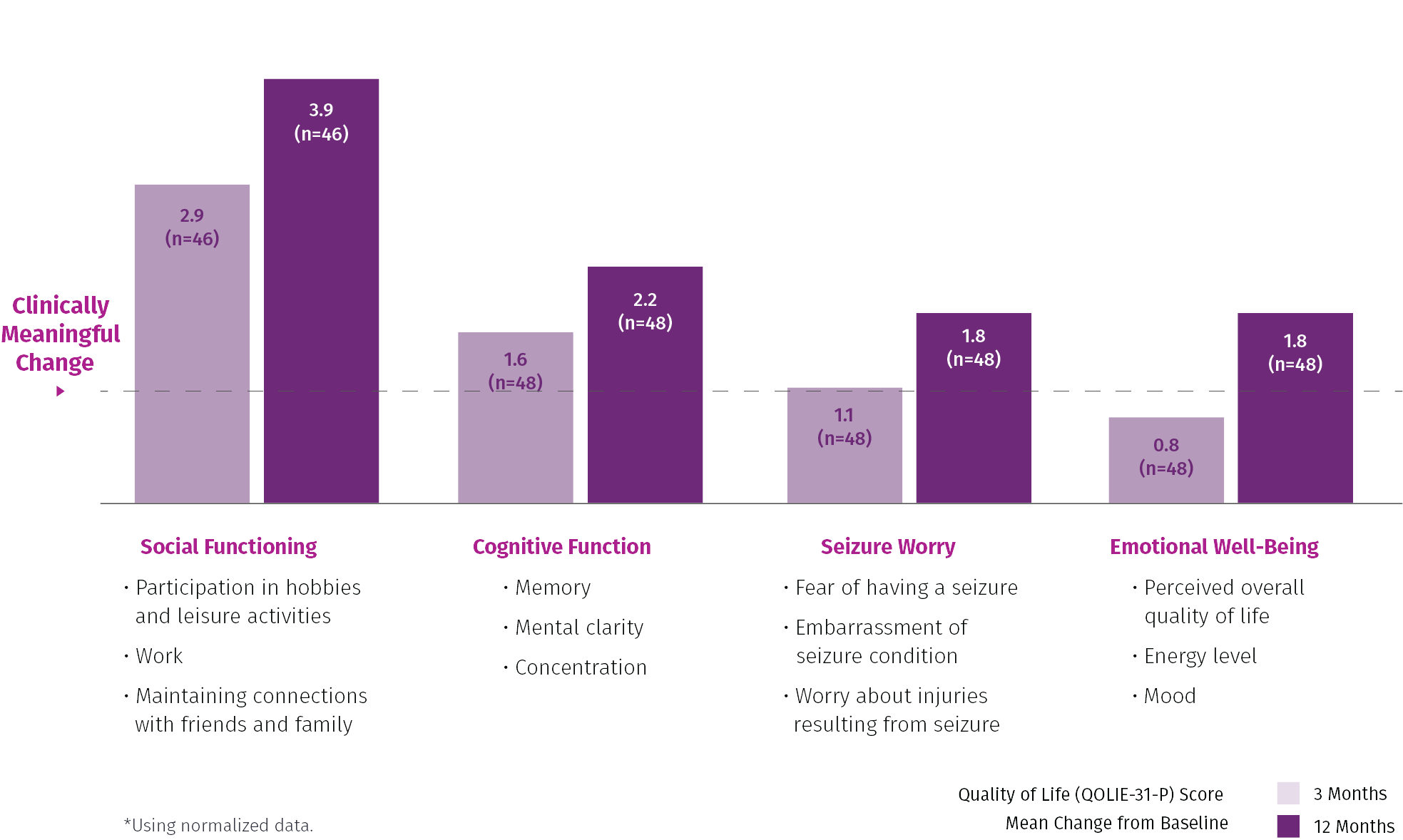
VNS Therapy™ demonstrates improvements in quality of life (memory, seizure worry and mood)
Improvements in quality-of-life confirmed across 2 prospective, multicenter, clinical trials (E-36) and (E-37)9
Implant the Future of Seizure Control
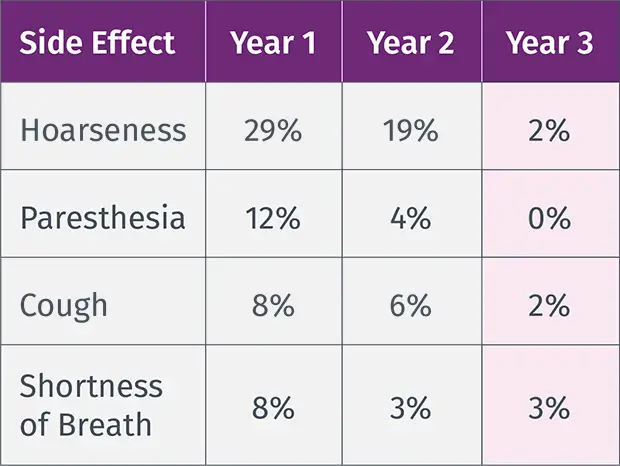
VNS TherapyTM Safety Profile
VNS Therapy is proven to be a safe adjunctive treatment and is well-tolerated by most patients without contributing to ASM toxicity.11,12
The most common side effects of VNS Therapy occur during stimulation and generally become less noticeable over time for most patients.11,13 Infection is the most common complication of the procedure.14
VNS Therapy™ has no neurotoxic effects or drug interactions13,14
Epilepsy (US) – The VNS Therapy System is indicated for use as an adjunctive therapy in reducing the frequency of seizures in patients 4 years of age and older with partial onset seizures that are refractory to antiepileptic medications.
VNS Therapy™ Safety Profile
Shortness of breath
Sore throat
Coughing
References: 1. Thurman DJ, Logroscino G, Beghi E, et al. Epilepsia. 2017;58(1):17-26. 2. Chen B, Choi H, Hirsch LJ, et al. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017;76:24-31. 3. Data on file. Survey report. LivaNova USA, Inc. Houston, TX. October 2021. 4. Alsfouk BAAA, Brodie MJ, Walters M, et al. JAMA Neurol. 2020;77(5):574-581. 5. Chen Z, Brodie MJ, Liew D, et al. JAMA Neurol. 2018;75(3):279-286. 6. Hamilton P, Soryal I, Dhahri P, et al. Seizure. 2018;58:120-126. 7. Data on file. CORE-VNS Clinical Study Report, LivaNova, USA, Inc. Houston, TX. June 2025. 8. Elliot RE, Morsi A, Tanweer O, et al. Effectiveness of vagus nerve stimulation over time: review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS >10 years. Epilepsy Behav. 2011;20(3):478-483. 9. Data on file. E-36, E-37 Pooled Calculations. LivaNova USA, Inc. Houston, TX. 2015. 10. Beaudreault CP, Chiang S, Sacknovitz A, et al. Association of reductions in rescue medication requirements with vagus nerve stimulation: results of long-term community collected data from a seizure diary app. Epilepsy Behav. 2024;159:1-7. 11. Ben-Menachem E. J Clin Neurophysiol. 2001;18(5):415 418. 12. Epilepsy Patient’s Manual for Vagus Nerve Stimulation, LivaNova, Inc., Houston, TX. 13. VNS Therapy System Epilepsy Physician’s Manual (US), April 2025, LivaNova USA, Inc. Houston, TX.

