
VNS Therapy™ reduces the frequency, duration, and severity of seizures and improves postictal recovery
In a study of 347 pediatric patients followed up for 24 months2:
.jpg?language=en-US)
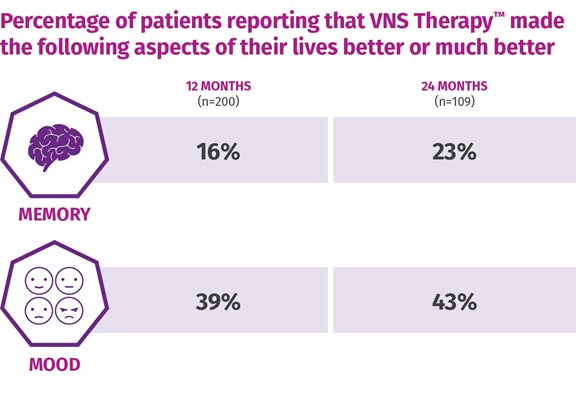
One-third of patients were considered "much improved" or "very much improved" following 12 months of adjunctive VNS TherapyTM (n=78), with sustained and improved scores continuing up to 24 months.5
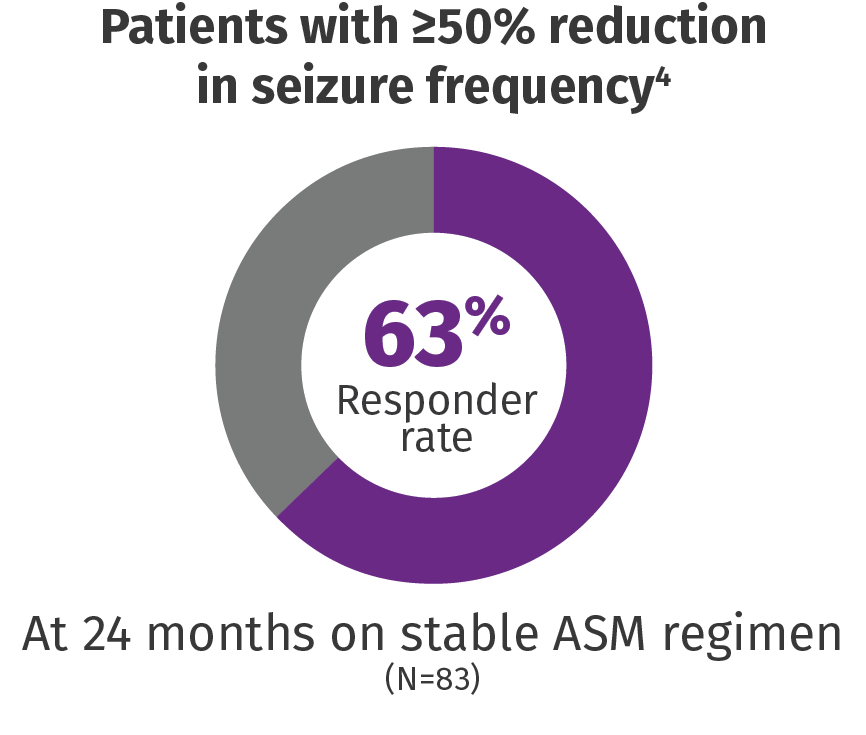
Based on the clinican's assessment of the patient's overall condition (CGI-I).
Seizure Reduction That Persists Over Time
76% mean seizure reduction in patients who completed 10 years of VNS TherapyTM 6
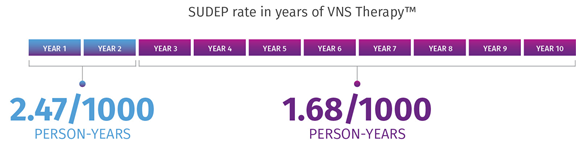
VNS Therapy may be associated with lower SUDEP rates.7
In a retrospective claims analysis, age-adjusted SUDEP rates decreased over time from years 1 to 2 (2.47/1000 person-years) to years 3 to 10 (1.68/1000 person-years).* A study on 277,661 person-years of up to 10 years of follow-up and 3689 deaths, including 632 cases of SUDEP, showed that long-term VNS Therapy is associated with lower SUDEP rates. The data suggest that SUDEP risk decreases during long-term follow-up of patients with refractory epilepsy receiving VNS Therapy.
*Limitations: This finding might reflect several factors, including ASM use, lack of a control group/seizure baseline, the natural long-term dynamic of SUDEP rate, attrition, and the impact of VNS Therapy. The role of each of these factors cannot be confirmed due to the limitations of study.
Implant the Future of Seizure Control
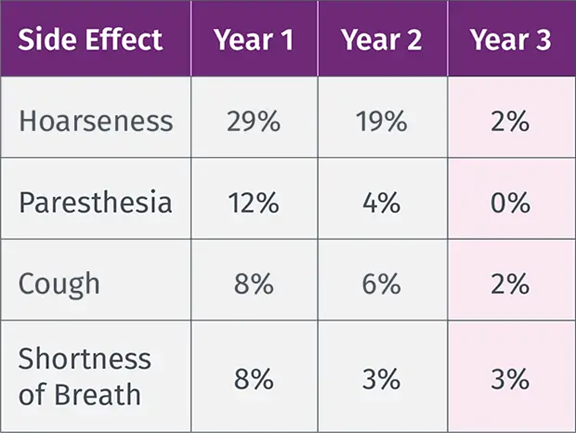
VNS TherapyTM Safety Profile
VNS Therapy is proven to be a safe adjunctive treatment and is well-tolerated by most patients without contributing to ASM toxicity. 8,9
The most common side effects of VNS Therapy occur during stimulation and generally become less noticeable over time for most patients.8,10 Infection is the most common complication of the procedure.9
VNS Therapy™ has no neurotoxic effects or drug interactions5,6
Epilepsy (US) – The VNS Therapy System is indicated for use as an adjunctive therapy in reducing the frequency of seizures in patients 4 years of age and older with partial onset seizures that are refractory to antiepileptic medications.
VNS Therapy™ Safety Profile
Shortness of breath
Sore throat
Coughing
References: 1. Data on file. Survey report. LivaNova USA, Inc. Houston, TX. October 2021. 2. Data on file. CORE-VNS Clinical Study Report, LivaNova, USA, Inc. Houston, TX. June 2025. 3. Lyons P, Wheless J, Verner R, et al. Vagus nerve stimulation in Lennox-Gastaut syndrome: twenty-four-month data and experience from the CORE-VNS study. Epilepsia. 2025;00:1-10. 4. Orosz I, McCormick D, Zamponi N, et al. Epilepsia. 2014;55(10):1576-1584. 5. Englot DJ, Hassnain KH, Rolston JD, Harward SC, Sinha SR, Haglund MM. Epilepsy Behav. 2017;66:4-9. 6. Elliot RE, Morsi A, Tanweer O, et al. Effectiveness of vagus nerve stimulation over time: review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS >10 years. Epilepsy Behav. 2011;20(3):478-483. 7. Ben-Menachem E. J Clin Neurophysiol. 2001;18(5):415 418. 8. Epilepsy Patient’s Manual for Vagus Nerve Stimulation, LivaNova, Inc., Houston, TX. 9. Morris GL, Mueller WM. Neurology. 1999;53(7):1731-1735. 10. Data on file, LivaNova USA, Inc., Houston, TX. 2025.

